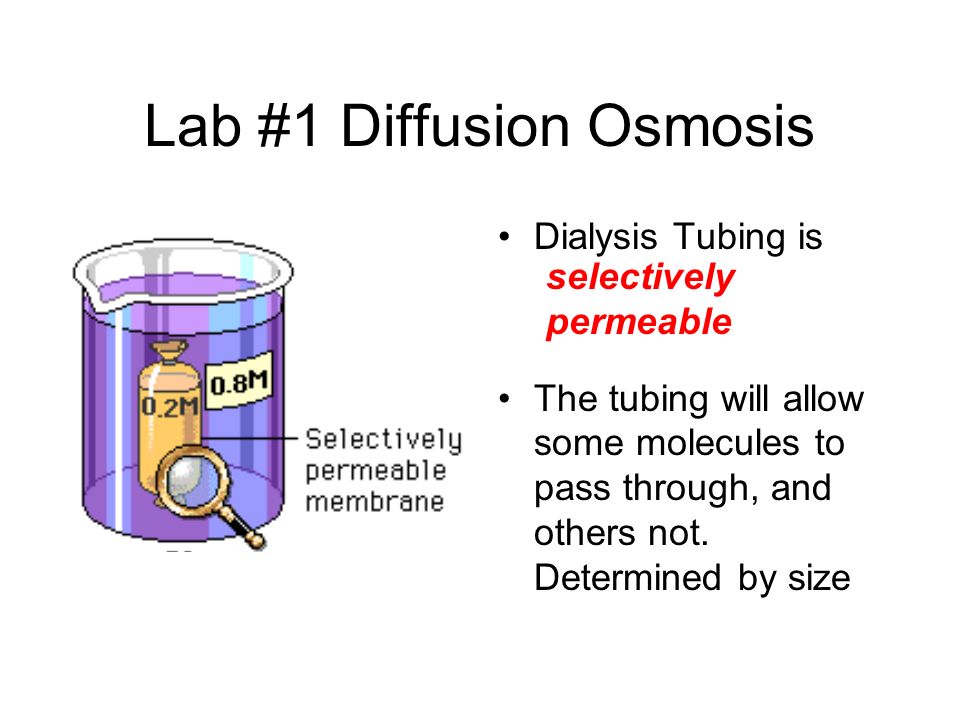
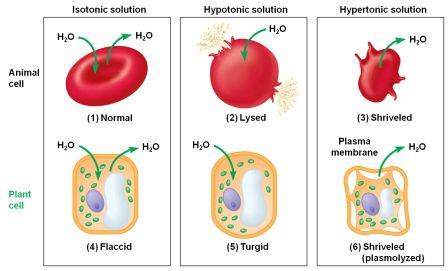
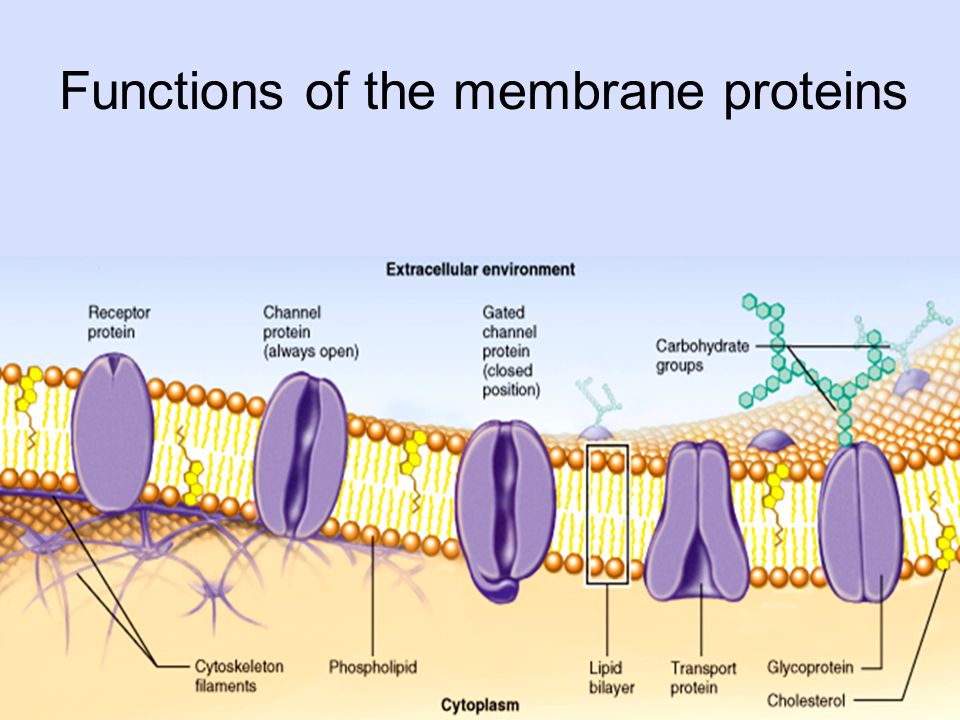
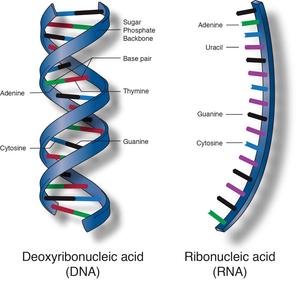
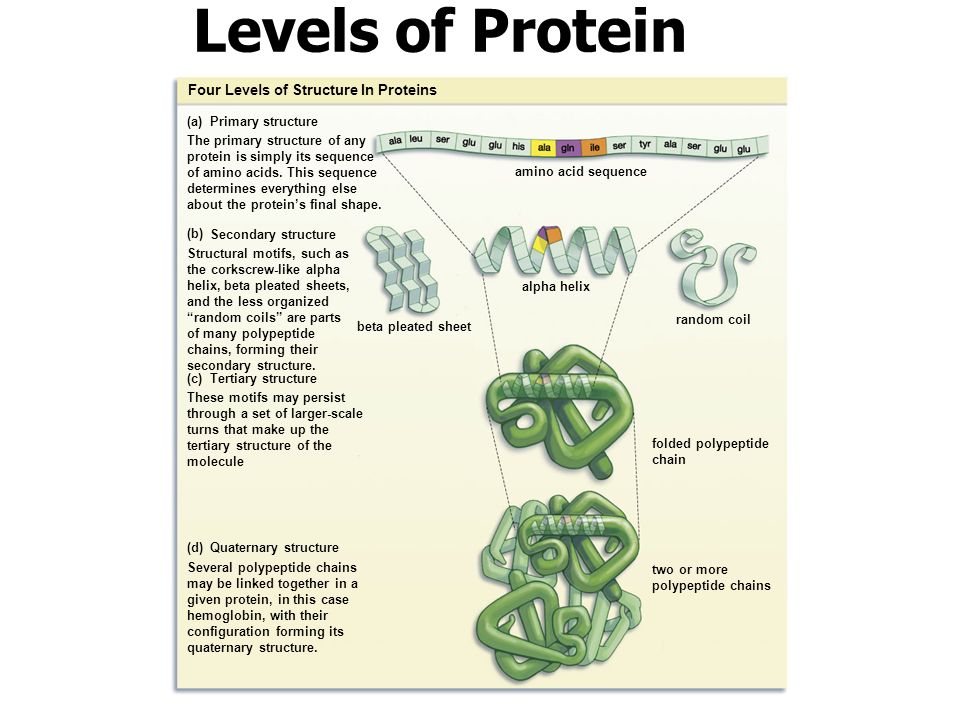
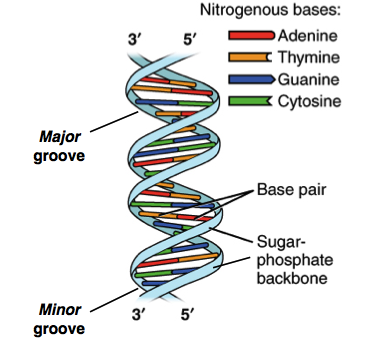
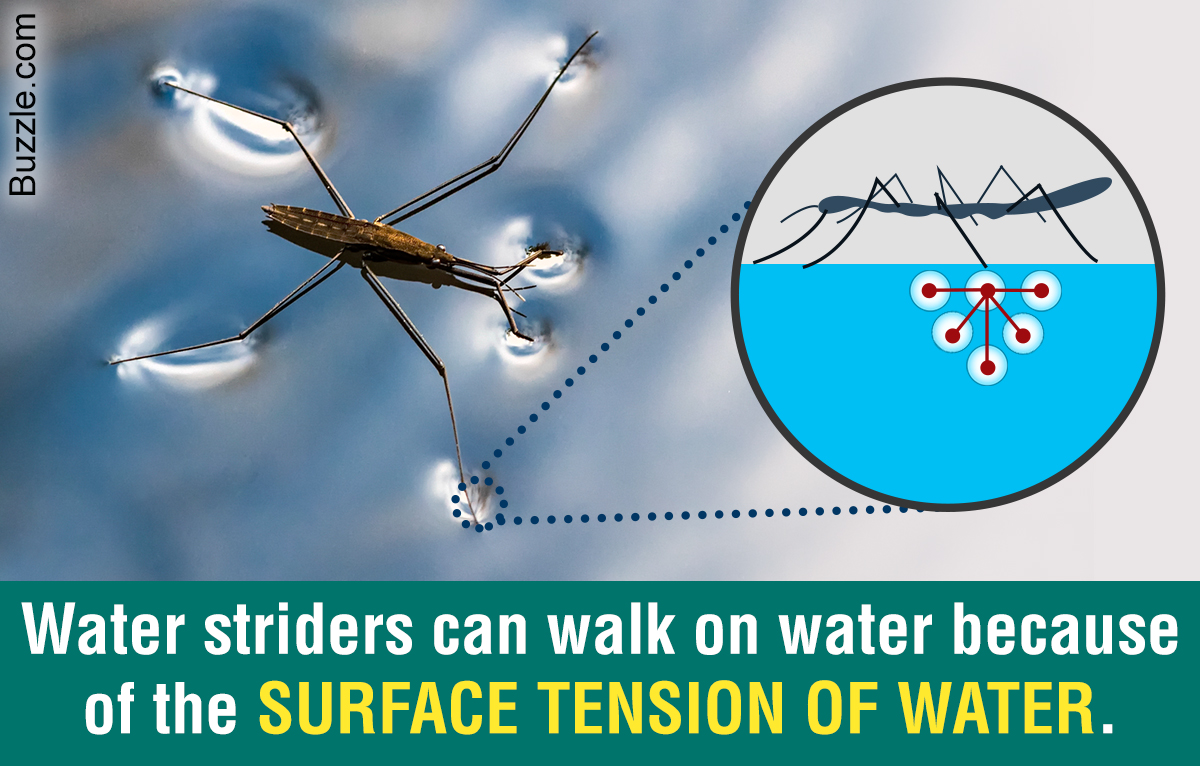
You will take notes on Enzymes. ( The names of enzymes usually end with ase)
The enormous of biochemical reactions occurring within cells is regulated by enzymes. Enzymes speed up chemical reactions, as well as control the rate at which reactions occur. They are globular protein molecules manufactured by each cell. More than 2000 enzymes have been recognized based on the chemical reactions they catalyze. All of them are structurally different.
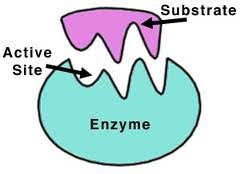
An enzyme recognizes a specific molecule called a substrate and binds to it. Some enzymes are so specific they only act on one substrate, while others can act on a class of substrate.
Enzymes can bring about changes to molecule to which it binds. The change usually involves the forming or breaking of a covalent chemical bond. Enzymes may split the substrate into two pieces, may add a chemical side group to the molecule, or may simply rearrange the bonds in the substrate.

Enzymes lower the activation energy by 1) providing a medium that is more favorable than the surrounding one. 2) By bringing the reactant into close contact. 3) They might add or remove a proton from the substrate , strain the substrate molecule's bond, or even form temporary covalent bond between the substrate and some part of the enzyme itself.







 (SA - volume ratio is surface area
(SA - volume ratio is surface area