Wishing you a fun and relaxing holiday. With most of the drama behind us let's make 2021 a great year. .
Hope I will get to meet you in the New Year.
Cheers to you and your family
Wishing you a fun and relaxing holiday. With most of the drama behind us let's make 2021 a great year. .
Hope I will get to meet you in the New Year.
Cheers to you and your family
Check Google classroom for work due on Thursday, December 10th.
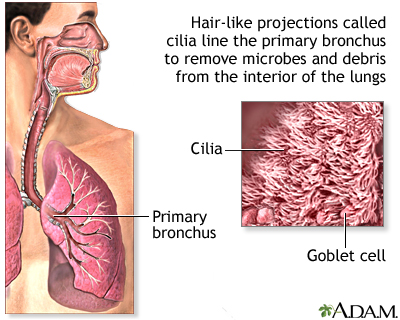
The class took notes on the functions of the organelles.
"Seeing is believing." The invention of the microscope made it possible to see cells and millions of tiny living organisms that are everywhere.
In 1665 Robert Hook used an early microscope to look at a thin slice of cork, the dead cells of oak bark. What he saw looked like rooms, which he called cells. The microscope was developed from eyeglass markers ideas in the late 1500 who realized that using several glass lenses magnified things.
The Light Microscope allows light to pass through a specimen and uses two lenses to form an image. Light waves are scattered as they pass through material. Therefore light microscopes can magnify up to about 1000 times.
Please upload the lab reports to the appropriate folder. You now have access to them in Google Classroom.
I hope you had a great Thanksgiving.
Period 1: I might be late to the Zoom meeting. Please log into Google Classroom for instructions.
Read through the notes and memorize the new vocabulary words. Doing so will help with the understanding of the chapter. The fill in the blank pages that correspond to the notes have been posted in Google classroom. Please attempt the questions before class tomorrow.
The first fifteen minutes of class will be spent explaining how to write a lab report.
We will continue to discuss the versatility of the hydrocarbon leading into the introduction of the macromolecules.
The 4 Macromolecules"
1) Carbohydrates
2) Protein
3) Lipids
4) Nucleic Acid
You will fill out the answers based on the demonstration of the lab. No formal lab write up is required for this lab.
The class will end with the PowerPoint presentation.
The information is posted in Google classroom. The folder to upload your work is also available.
Video link for Cell transport
Water Poisoning: Analogy based on the true story of Hold you wee for a Wii
Many enzymes require non-protein substances to function. Without them
the enzymes would not work efficiently. There are two types of these
enzyme helpers:
1) Cofactor: inorganic molecules, minerals, usually metal ions. For
instance magnesium is important in reactions where a phosphate group is
transferred. One example of this is reversible reaction of ATP.
These cofactors can turn enzymes on and off or modify the rate at which
enzymes work. Iron is another example. Iron is an integral part of
hemoglobin's ability to transport oxygen,
2) Coenzymes: organic compounds like vitamins. Coenzymes bind to
specific site on a protein molecule and provides chemical functions that
a protein alone cannot provide. You body can make the necessary
enzymes, but not the necessary minerals and vitamins which must be
included in the diet.
Remember that enzymes usually end in ase; catalase, sucrase.
The enormous of biochemical reactions occurring within cells is
regulated by enzymes. Enzymes speed up chemical reactions, as well as
control the rate at which reactions occur. They are globular protein
molecules manufactured by each cell. More than 2000 enzymes have been
recognized based on the chemical reactions they catalyze. All of them
are structurally different
An enzyme recognizes a specific molecule called a substrate and binds to
it. Some enzymes are so specific they only act on one substrate, while
others can act on a class of substrate.
Enzymes can bring about changes to molecule to which it binds. The
change usually involves the forming or breaking of a covalent chemical
bond. Enzymes may split the substrate into two pieces, may add a
chemical side group to the molecule, or may simply rearrange the bonds
in the substrate.
Enzymes lower the activation energy by 1) providing a medium that is
more favorable than the surrounding one. 2) By bringing the reactant
into close contact. 3) They might add or remove a proton from the
substrate , strain the substrate molecule's bond, or even form temporary
covalent bond between the substrate and some part of the enzyme itself.
In an exergonic reaction energy is released so the reactants have more energy than the products. For example: Cellular Respiration. See equation below.
In an endergonic reaction the product has more energy than the reactant. For example; Photosynthesis. See the equation below.


The review was necessary to prevent having a repeat of the errors encountered in the Nutrient Lab. You can again review the Lab Write-up Format posted in a separate tab on this blog.
The folder for Chapter 4 uploads is available on Google classroom.
The test will be on Monday, October 12, 2020Chapter 4 Packet is due on Friday.
The Study Guide for the chapter 4 multiple choice test is listed in Google Classroom and the school home work page.

Video links: An Overview of the Cell Structure
Amoeba Sisters: Introduction to the Cell
Here is the link to the video clip shown in 3rd period.
Today's class we continued with the functions of the organelles.
The Animal Cell

"Seeing is believing." The invention of the microscope made it possible
to see cells and millions of tiny living organisms that are everywhere.
In 1665 Robert Hook used
an early microscope to look at a thin slice of cork, the dead cells of
oak bark. What he saw looked like rooms, which he called cells. The
microscope was developed from eyeglass markers ideas in the late 1500
who realized that using several glass lenses magnified things.
The Light Microscope allows light to
pass through a specimen and uses two lenses to form an image. Light
waves are scattered as they pass through material. Therefore light
microscopes can magnify up to about 1000 times.

Electron Microscopes use beams of
electrons focused my magnetic fields. These offer higher resolutions
than light microscope. These are used to only examine non-living cells
and tissues. The samples are chemically preserved so that they can be
examined in a vacuum. The electrons are placed in a vacuum to prevent
them from being scattered.
Click on the link below to see how electron microscopes work:
The study guide is posted on Google classroom and on the home work page.
All the pages of chapter 3 are due on Monday, September 28th. See you tomorrow for the Nutrient Testing Lab.

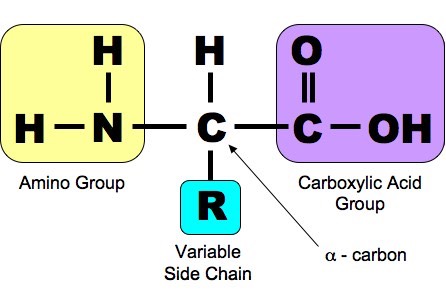
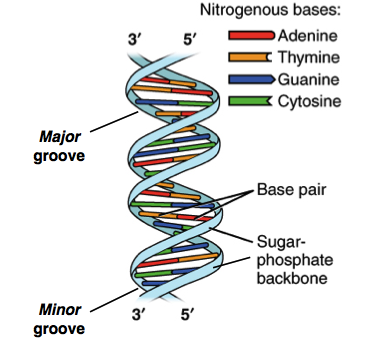 The DNA Molecule
The DNA Molecule